Case Report
Secondary Onychomycosis Development after Cosmetic Procedure-Case Report

Mariusz Dyląg1*, Emilia Flisowska1, Patryk Bielecki2, Maria Kozioł-Gałczyńska3 and Weronika Jasińska4
1Department of Genetics, Institute of Genetics and Microbiology, University of Wroclaw, Przybyszewskiego Street 63/77, 51-148 Wroclaw, Poland
2Voivodeship Sanitary-Epidemiological Station in Olsztyn, Zolnierska 16, 11-041 Olsztyn, Poland
3Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, Chalubinskiego Street 1, 50-368 Wroclaw, Poland
4Department of Life Science, Faculty of Natural Science, Ben-Gurion University of the Negev, Beer Sheva, Israel
*Address for Correspondence: Mariusz Dyląg, Department of Genetics, Institute of Genetics and Microbiology, University of Wroclaw, Przybyszewskiego Street 63/77, 51-148 Wroclaw, Poland, Tel: + 0048713756351; +48512614050; Email: [email protected]
Dates: Submitted: 16 March 2016; Approved: 24 April 2017; Published: 25 April 2017
How to cite this article: Dyląg M, Flisowska E, Bielecki P, Kozioł-Gałczyńska M, Jasińska W. Secondary Onychomycosis Development after Cosmetic Procedure-Case Report. J Clin Med Exp Images. 2017; 1: 037-045.
DOI: 10.29328/journal.jcmei.1001007
Copyright License: © 2017 Dyląg M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
The authors describe the unusual case of subungual onychomycosis, due to fluconazole and itraconazole resistant Candida albicans after using the hybrid and acrylic lacquers and nail tips. The etiology of these atypical changes was supported by isolation of the fungus from the nail lesions, and its consistent identification by means of morphological and molecular diagnosis. In the presented case, topical treatment with ciclopirox 8% nail lacquer allow to fight the pathogenic fungus but did not restore the natural appearance of the nails.
INTRODUCTION
Changes in the fingernails as well as in toenails may have different etiology what has been extensively described [1]. To disease entities associated with nails are included mainly nail psoriasis, lichen planus, fungal and bacterial infections. Among the less common cases are trachyonychia, paronychia, nail tumors or ingrown toenail. In recent years, more and more attention is paid to manifesting in nail plates some psychosomatic disorders or systemic diseases [1,2]. Moreover, changes in the nail plates can be induced by certain drugs or be the result of the trauma. While the first of these usually are connected with all the nails (for drug taken orally), but the second mentioned usually affect only one nail [1,3]. The already mentioned fungal and bacterial infections can be both primary as well as secondary infection what was previously reviewed [3,4]. Fungal infections of fingernails as well toenails are called onychomycosis and those are more common than infections caused by bacteria. It is estimated that onychomycosis affects 3-8% of the human population of highly developed countries. Wherein it should be emphasized that onychomycosis of toenails is about 4-7 times more common than the onychomycosis of the fingernails [5-8]. Described by us clinical case is an example of a secondary fungal infection, which most likely developed as a result of multiply procedures performed using the hybrid and acrylic lacquers and the nail tips.
CASE REPORT
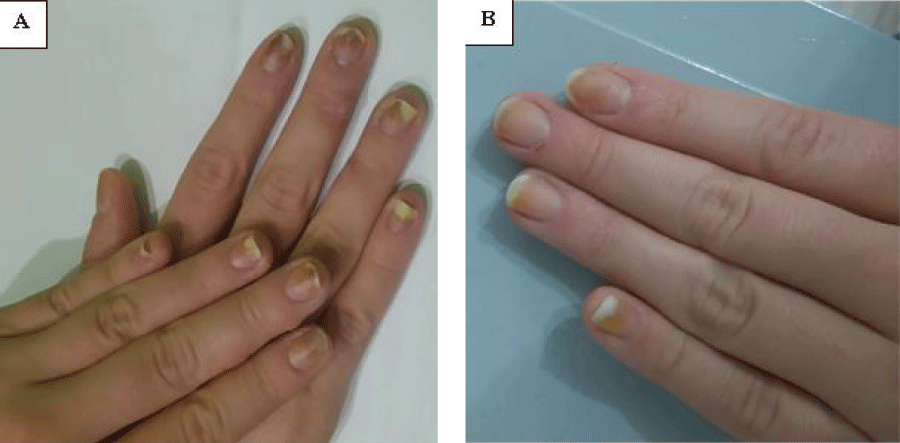
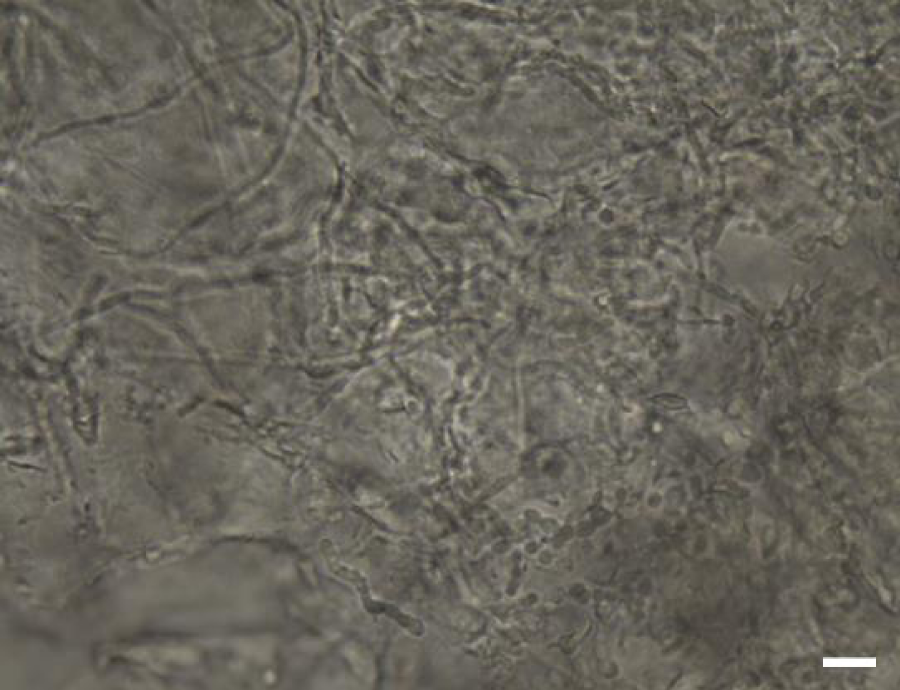
In June 2016, a 32 year old women was examined for the fungal infection of the finger nails. The first changes patient observed in May 2016 after removing the hybrid nail tips. Changes consisted of all the nail plates of the right and left hand, and were characterized by the same clinical picture. Several nail plates were changed only in distal part of plate in about 20-30%. Within the plates we could observed only white-yellow discoloration. Except such changes in case of the fourth and fifth finger in both right and left hand distally were observed the small empty spaces under the nail plates. The surface of the nail plate was neither eroded nor deformed. The patient was immunocompetent and in a general good condition. Neither systemic disease nor any use of immunosuppressive drugs were evidenced in the patient’s history. After dermatological consultation, mycological examination was performed. Direct examination of the nail scrapings treated with dimethyl sulfoxide (DMSO, Sigma-Aldrich, Poland) and 10% potassium hydroxide (KOH, Sigma-Aldrich, Poland) did not show any fungal structures which could be indicate on the fungal infection. Similarly all the mycological cultures were negative. The women was examined second time in August 2016. As it was recommended by dermatologist between the first and second visit the woman had stopped the cosmetic procedures. However, patient had not cut the detached nail plates. Moreover on this time any treatment was advised. During the second visit could be observed deepening changes in the most of the nail plates. Despite the white-yellow discoloration in the distal parts of the nail plates we could also see brown-yellow stains especially located in the deeper parts of the nails. Additionally, subungual hyperkeratosis and deepening onycholysis was observed (Figure 1a) comparing to the first time when the patient was examined. The changes consisted even 50-60% of the nail plate as it was visible especially in the case of second and firth finger nail plates. The clinical picture resembled a distal and lateral subungual onychomycosis (DLSO). This time the direct examination in KOH with DMSO was positive. The microscopic observations allowed to show both the presence of true hyphae as well pseudohyphae and blastoconidia (Figure 2). In the same way as for the first time when patient was examined mycological cultures were carried out both on Sabouraud glucose agar (SGA, Biomaxima, Poland) with chloramphenicol and gentamicin as well on SGA with cycloheximide (all reagents obtained from Sigma-Aldrich, Poland). Additionally, the nail scrapings also were inoculated on dermatophyte test medium (DTM, Sigma-Aldrich, Poland) with chloramphenicol and gentamicin but without cycloheximide. All the cultures were incubated aerobically at 28°C for up to 21 days. Starting from the 2nd day post inoculation, white to cream colored, smooth and butyrous colonies were observed. All the colonies were one of the particular type and grew on all the used media. Ability to grow on SGA with cycloheximide allow to conclude about natural resistance to this compound what was an important criterion in the further species identification of the isolated strain. The character of growth on DTM with typical for non-dermatophyte fungi acidification also confirmed our observations. After 3-4 days post inoculation all the colonies reached maturity. The micromorphology observed under the light microscope (Carl Zeiss, Jena) was the same for colonies growing on the three different above mentioned media. In each time were visible globose to ovoid cells (3-6μm in diameter) multilaterally budding (data not shown).
Figure 1: The clinical picture of the fingernails before antifungal therapy (A), and after a 2-month topical therapy with ciclopirox 8% nail lacquer (B).
Figure 2: Fungal structures visualized by direct examination of the nail scrapings after 10% KOH and DMSO (dimethyl sulfoxide) treatment, magnification 400x, scale bar: 10μm.
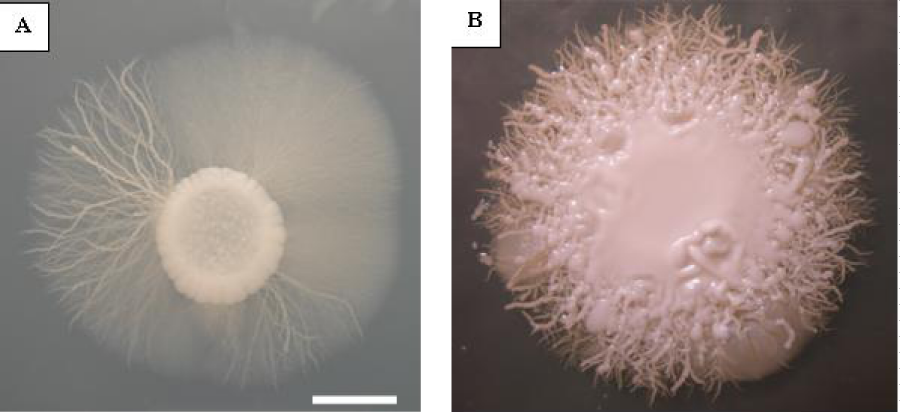
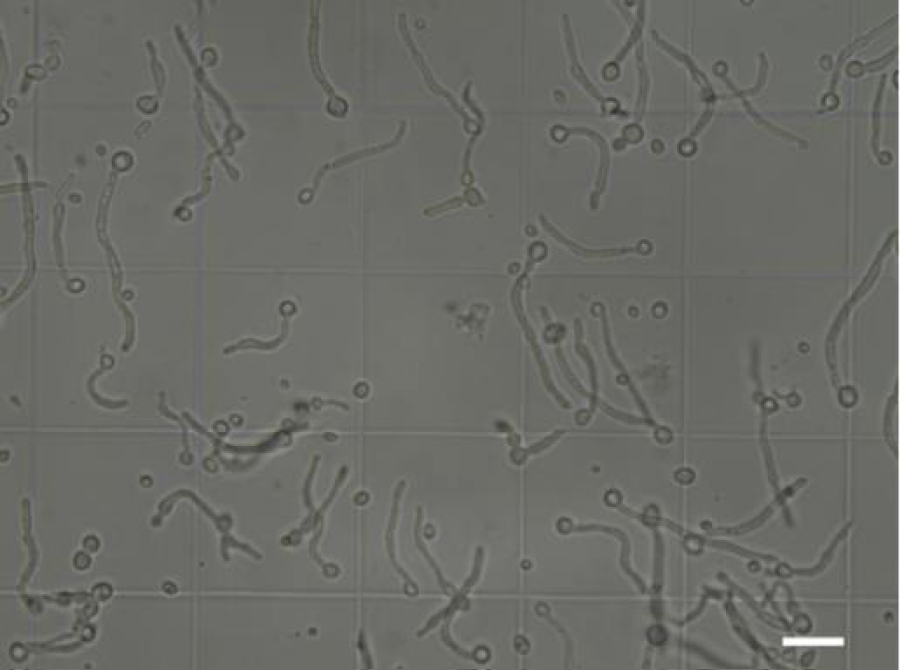
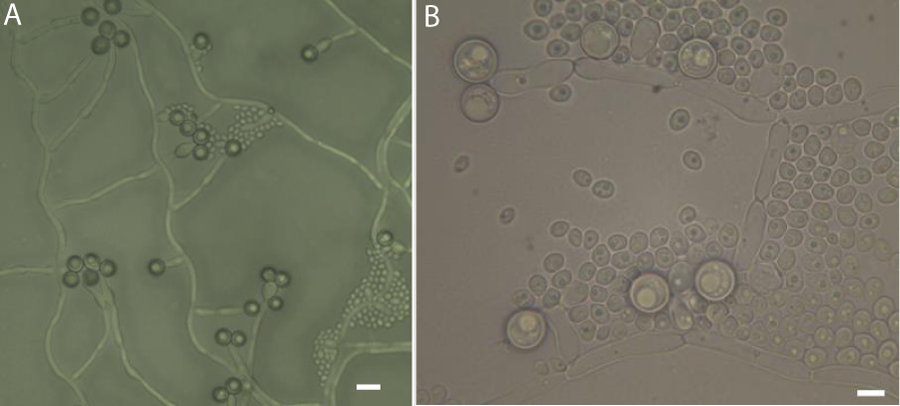
Species identification was performed for this isolate according to described in the literature procedures commonly used in classical mycological diagnostics (McGinnis, 1980). To achieve this aim isolate were subcultured on the chromogenic medium-CHROMagar™ Candida (bioMérieux, Poland) on which the single colonies have blue-green color. Colonies were also subcultured on a RAT (Rice Agar Tween), Christensen’s urea, spider (SM) and synthetic low-ammonium-dextrose (SLAD) media, all prepared as described elsewhere [9,10]. As we could observe on a Christensen medium our strain did not show ability to urea hydrolysis (data not shown). The specific character of growth on SM and SLAD media and the positive result of the Germ Tube test (GT) allow to conclude that our isolate has very strong ability to the phenotype switching and to grow both in yeast as well in mycelial form depending on culture/test conditions (Figure 3 a,b and Figure 4). The characteristic structures were also visible for the strain which grows on the RAT medium in 25oC. In the case of this medium the most important was to show ability to the chlamydospore formation which on average were 6-12μm in diameter and were thick-walled comparing to about two times smaller blastoconidia (Figure 5 a,b). Evaluation of the macro- and micromorphology characteristic for this strain depending on used medium and also results of the physiological and biochemical tests unequivocally indicated that we are dealing with Candida albicans (Robin) Berkhout.
Figure 3: Macroscopic appearance of Candida albicans (isolated from fingernails) culture on synthetic low-ammonium-dextrose (SLAD) medium (A) post 10-day incubation at 37oC, scale bar: 5 mm and (B) on spider medium (SM) after 7-day incubation at 37oC, scale bar: 10 mm.
Figure 4: The result of Germ Tube test (GT) performed in bovine serum at 37oC for 5 hours for clinical strain isolated from fingernails, magnification 400x, scale bar: 10μm.
Figure 5: Microscopic morphology of Candida albicans (clinical strain) growing on rice agar tween (RAT) medium after 7-day incubation at 25oC, (A) magnification 400x; (B) magnification 1000x, scale bar: 10μm.
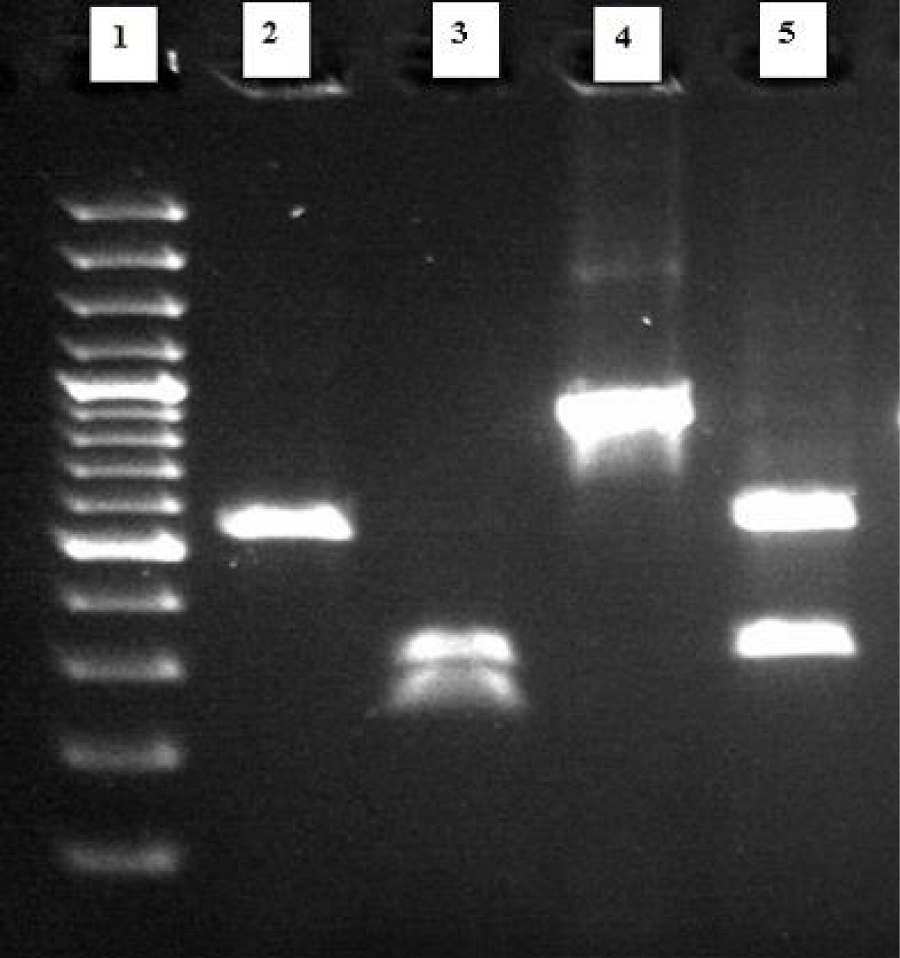
To confirm the diagnosis conducted by classical methods, the molecular identification was performed. This involved genome DNA extraction from pure culture (obtained from single colony) using commercially available DNA isolation kit-Genomic Mini AX Yeast (A&A Biotechnology, Poland) and performed according to the producer instruction. Obtained chromosomal DNA was used as a template DNA both in PCR-RFLP (Polymerase Chain Reaction-Restriction Fragment Length Polymorphism) method as well Multiplex F-A-N PCR technique. As it was previously shown the first technique based on a one-enzyme PCR-RFLP assay allow to identify in the same time six medically important Candida species including C. albicans [11]. For this reason also was used by us. In the case of this assay were used standard primers (obtained from Genomed, Poland) (Fermentas, Poland). The composition of the reactions mixtures and conditions both for PCR as well as for digestion reactions were as described earlier ITS1 (forward) and ITS4 (reverse) which allow to amplify the ITS1-5.8S-ITS2 region of fungal rRNA genes. Then, the obtained PCR products were digested using MspI (HpaII) a restriction enzyme [11]. As a quality control was used Candida glabrata ATCC 90030 a reference strain. Obtained PCR products for the clinical isolate and a reference strain were equal to 535 bp and 871 bp, respectively. The length of the restriction fragments were as follows: 557 bp and 314 bp for C. glabrata ATCC 90030 and for the clinical isolate 297 bp and 238 bp (Figure 6).
Figure 6: Restriction digestion of PCR (Polymerase Chain Reaction) products of the clinical isolate and the reference strain with the enzyme Msp I (Hpa II). Lanes: 2) clinical isolate (Candida albicans) PCR product (535 bp); 3) digested product (297 bp and 238 bp); 4) reference strain (Candida glabrata ATCC 90030) PCR product (871 bp); 5) digested product (557 bp and 314 bp); Lane 1 - molecular size marker 100-3000 bp (Bio-Rad).
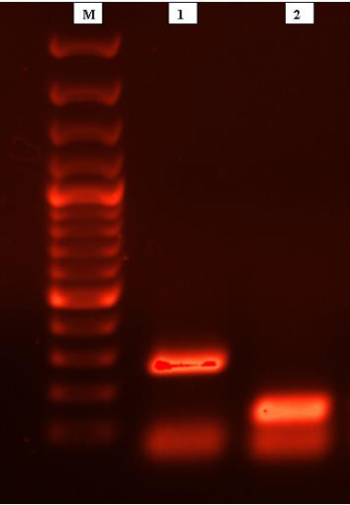
For the second method were used standard primers (obtained from Genomed, Poland) AFUM1-AFUM2, CALB1-CALB2, and CN5-CN4 as described previously (Luo and Mitchell, 2002). These primers allow to identify simultaneously three fungal pathogens in a single reaction: Aspergillus fumigatus, C. albicans, and C. neoformans, respectively. As a positive PCR control was used genomic DNA (as a matrix) isolated from Cryptococcus neoformans H99, a reference strain (obtained from Joseph Heitman). The reaction mixture composition and conditions for PCR reaction were the same as described previously [12]. The amplicons size in case of our clinical isolate and for the reference strain were equal to 273 bp and 136 bp, respectively (Figure 7). All the PCR products and restriction fragments were separated by electrophoresis in a 1.5% agarose gel with 0.5μg of ethidium bromide (Sigma-Aldrich, Poland) per mililitre and 1x concentrated TBE (Tris-borate-EDTA) buffer. Then amplicons were visualized on a UV transilluminator (Bio-Rad). Overall, based on the phenotypic and molecular data (size of PCR amplicons and restriction fragments) the fungus isolated from the fingernails was identified as C. albicans (Robin) Berkhout.
Figure 7: Specific amplicons generated according to multiplex PCR performed with genomic DNA from clinical isolate-line A (273 bp) and reference strain (Cryptococcus neoformans H99) - line B (136 bp); lane M-molecular size marker 100-3000 bp (Bio-Rad).
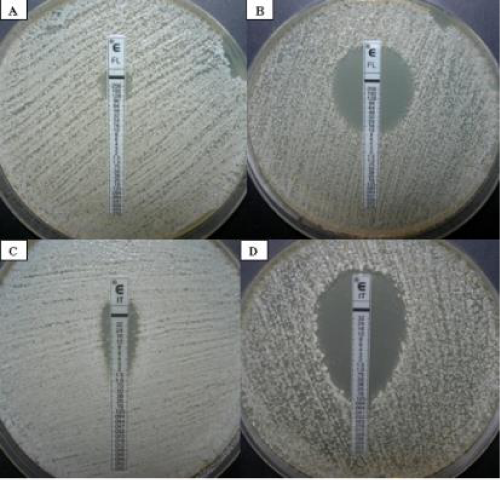
The susceptibility testing of the obtained clinical isolate toward the chosen drugs was performed with the Etest method, according to the manufacturer`s procedure [13]. An important modification was that the assay was carried out on YNB (Yeast Nitrogen Base without amino acids, Becton Dickinson and Company, USA) medium instead of RPMI 1640 (Roswell Park Memorial Insitute 1640, Sigma-Aldrich, Poland) medium, which is recommended in the original protocol [13]. This was forced by the fact that we checked susceptibility also toward tacrolimus (FK506, Sigma-Aldrich, Poland) and rhodamine 6G (R6G, Sigma-Aldrich, Poland) used solely or in a combination with fluconazole or itraconazole (both from Sigma-Aldrich, Poland). Among the tested compounds were only fluconazole and itraconazole as the drugs most commonly used in the treatment of onychomycosis especially caused by yeast-like fungi [14,15] and because the Etests strips are commercially available for them. The MIC (Minimal Inhibitory Concentration) values for these drugs in the case of our isolate were equal to 128μg/ml for fluconazole and 3μg/ml for itraconazole (Figure 8 a,c). According to European Committee on Antibiotic Susceptibility Testing (EUCAST, 2015) [16] such results clearly indicate that this strain was resistant toward the both drugs. In view of the manifested by the isolated strain in vitro resistance to these two triazoles additional tests were carried out with FK506 and R6G to look more closely at the problem. Studies with tacrolimus allow to conclude that this phenotype is reversal when fluconazole as well itraconazole is used in a combination with tacrolimus at concentration equal to 5μM (Figure 8 b,d).
Figure 8: Candida albicans (clinical isolate) susceptibility to fluconazole and itraconazole tested solely or in combination with tacrolimus (FK 506); (A) fluconazole used solely: MIC=128μg/ml; (B) fluconazole in combination with FK 506: MIC=12μg/ml; (C) itraconazole used solely: MIC=3μg/ml; (D) itraconazole in combination with FK 506: MIC=0.125μg/ml.
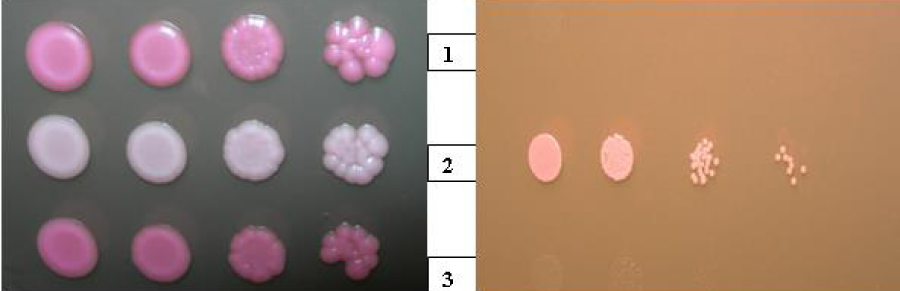
Despite the resistance to fluconazole and itraconazole, our isolate also shown the lowest susceptibility to R6G. Comparing our clinical strain with susceptible to both azole drugs C. albicans ATCC 90028 and C. glabrata ATCC 90030 reference strains this isolate was able to grow in the presence of R6G at concentration equal to 80μg/ml (Figure 9 a) while the susceptible strains stop growing in the presence of this compound at concentration from 8 to 15μg/ml. Moreover, in the presence of rhodamine 6G in a medium at concentration equal to 3μg/ml (Figure 9 b) or even 10μg/ml colonies of this strain remained white while colonies of the other strains took pink coloration at the same conditions. Intensity of the colony color was increasing with the growing concentration of rhodamine 6G in an YNB medium.
Figure 9: Differences in susceptibility to rhodamine 6G between Candida albicans clinical strain (isolated from fingernails, line 2), C. albicans ATCC 90028 (line 1) and Candida glabrata ATCC 90030 (line 3) reference strains evaluated by serial dilutions technique (spot tests).
Considering the in vitro resistance toward fluconazole and itraconazole and a lack of information about the susceptibility to terbinafine it was decided to treat this infection with ciclopirox (Penlac® Nail Lacquer). The 8% nail lacquer was used topically once daily. The infection was eradicated after 3-month of a topically treatment with a ciclopirox. It was confirmed (in two independent repetitions separated temporally) by mycological examination which included negative direct microscopy and culture. Although candidiasis has been fighted and we could not observe any relapse of the infection during a 4-month follow-up period the treatment was not fully successful. Though the clinical picture has significantly improved and the fingernails were free of any fungal structures there still were present discolorations and the natural appearance of the nail plates was not restored (Figure 1b).
DISCUSSION
Shown in the case of isolated C. albicans strain in vitro resistance to fluconazole and itraconazole is not uncommon. There are different molecular mechanisms of azole resistance that were reviewed in the literature [17]. The both triazoles similarly as rhodamine 6G are well known substrates for Saccharomyces cerevisiae Pdr5p [18] and Cdr1p which is its homologue in the cells of C. albicans [19]. The observed simultaneously resistance to both tested triazoles and rhodamine 6G allows rather to conclude about resistance phenotype generated by efflux pumps than by genes associated with the ergosterol synthesis as even ERG3 or ERG11 [17]. An additional proof of the existence of this particular phenotype in the case of our isolate are the results of studies with tacrolimus. This compound is a known inhibitor of the calcineurin a pathway. It can also reverse resistance phenotype generated by localized in the plasma membrane of S. cerevisiae Pdr5 protein or its homologue Cdr1p as in the case of C. albicans through direct binding to the transmembrane domain 10 (TMD10) [20]. It blocks the ability to cell detoxification, what was shown by flow cytometric assays and restores susceptibility to azole drugs and other compounds that are substrates for Pdr5/Cdr1 protein [21]. This was clearly visible in the tests with tacrolimus, which was used only in a concentration equal to 5μM restored the phenotype of sensitivity to these two compounds. Described for this strain phenotype will be the subject of further more specific studies.
Despite the resistance phenotype toward both triazoles the treatment was effective with ciclopirox 8% nail lacquer. Although Etest strips for this drug are not available, it was used because of its broad spectrum of antifungal activity and strong fungicidal properties [14]. Similarly as for ciclopirox also for terbinafine Etests strips are not available, but according to the literature this drug is not recommended for the treatment of onychomycosis caused by yeast-like fungi [15]. Although terbinafine show broad and strong fungicidal effects against dermatophytes it has lower fungistatic activity against Candida species comparing to the azoles [15]. Although therapy with ciclopirox allowed to completely eradicate the infection with no further relapse, however the changes in the structure of the nail plate remained and the natural color of the fingernails was not restored. It should be concluded that after successful antifungal therapy what this is extremely urgent in case of this patient is proper therapy which restore the fingernails a health. This problem was widely described previously [22]. Based on an interview with the patient and the characteristic clinical picture of the nails on the day of the first visit it can be concluded that the repeated use of hybrid and acrylic lacquers and the nail tips led to the specific devastation of the nails. As patient revealed by over a year it was performed such cosmetic procedure at least several times in various beauty salons. The patient also drew attention to the fact that usually after such surgeries an inflammation of periungual shafts was observed. On the day of the first visit in June 2016 clinical picture for all the ten fingernails were very similar and in this case was predominated by the underling nail pathology rather than fungal infection. A characteristic symptom was onycholysis occurs in the distal part of the fingernails, discoloration and slightly eroded surface. Therefore it is very likely that the observed changes were a result of exposure of fingernails to quite aggressive chemicals. Undoubtedly to this type of preparations we can include acetone and toluene sulfonamide formaldehyde commonly used in beauty salons. The latter especially is now well known factor which causes the allergic contact dermatitis [23,24]. During the second visit in August 2016 it could be concluded that this time we are dealing with a secondary fungal infection, which direct reason was most likely earlier carried out cosmetic treatments. Among all the observed changes in the fingernails particularly favorable factor for the fungal infection development was onycholysis. It was many times underlined that onycholysis creates a small pocket of dead space that fungi can colonize and develop infection. Moreover, it is also worth to mention that the very process of removing the tips is an important risk factor for the nails what was previously reviewed [3,22,24]. Furthermore, acetone in which nails usually are soaked even 10-15 minutes to remove the nail tips and also different acrylate glues or exposure to the UV lamp can provide to different changes in the nail plates. Such changes described elsewhere may qualify for the development of a fungal or bacterial infection [24-26].
In summary in this paper was described onychomycosis caused by in vitro resistant to azole C. albicans, in case of which the most probable cause were cosmetic procedure carried out earlier. Moreover, in this case report we also pay attention to the potential risks associated with the use of improper preparations or inappropriately beauty treatments.
REFERENCES
- Sacchidanand S, Savitha AS. Nail & its disorders, 1st edition, Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India. 2013; 84-235.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015; 6: 67-74. Ref.: https://goo.gl/THPZLj
- Vlahovic TC. A closer look at cosmetic solutions for nails. Podiary today. 2014; 27: Ref.: https://goo.gl/ezOQkb
- Hay RJ, Baran R. Onychomycosis: A proposed revision of the clinical classification. J Am Acad Dermatol. 2011; 65: 1219-1227. Ref.: https://goo.gl/qRHBHP
- Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992; 126: 23-27. Ref.: https://goo.gl/RLU4DA
- Heikkala H, Stubbs S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995; 133: 699-703. Ref.: https://goo.gl/qog4cg
- Gupta AK, Jain HC, Lynde CW, Watteel GN, Summerbell RC. Prevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologist’s offices in Ontario, Canada-a multicenter survey of 2001 patients. Int J Dermatol. 1997; 36: 783-787. Ref.: https://goo.gl/yLiFXS
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014; 28: 1480-1491. Ref.: https://goo.gl/DhqExv
- McGinnis MR. Chapter 5: Yeast identification, Academic Press Inc, New York, USA. Laboratory handbook of medical mycology. 1980; 337-410.
- Kane J, Summerbell R, Sigler L. Laboratory handbook of dermatophytes: A clinical guide and laboratory handbook of dermatophytes and other filamentous fungi from skin, hair and nails, Star Publishing Company, Belmont. USA. 1997; 314-331.
- Mirhendi H, Makimura K, Khoramizadeh M, Yamaguchi H. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nihon Ishinkin Gakkai Zasshi. 2006; 47: 225-229. Ref.: https://goo.gl/tkc0DT
- Luo G, Mitchell TG. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J Clin Microbiol. 2002; 40: 2860-2865. Ref.: https://goo.gl/3U64Ch
- AB BIODISK. Etest technical guide 10 (ETG 010): antifungal susceptibility testing of moulds, AB BIODISK. Solna, Sweden. 1999.
- Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003; 149: 296-305. Ref.: https://goo.gl/fSdb2z
- Bueno JG, Martinez C, Zapata B, Sanclemente G, Gallego M, et al. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis, Clin Exp Dermatol. 2010; 35: 658-663. Ref.: https://goo.gl/wTtjZf
- European Committee on Antimicrobial Susceptibility Testing. Antifungal agents. Breakpoint tables for interpretation of MICs. version 8.0. 2015.
- Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, et al. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front Microbiol. 2016; 7: 2173. Ref.: https://goo.gl/HW8Kq8
- Ernst R, Klemm R, Schmitt L, Kuchler K. Yeast ATP-binding cassette transporters: Cellular cleaning pumps. Method Enzymol. 2005; 400: 460-484. Ref.: https://goo.gl/WmsBfi
- Golin JA, Ambudkar SVB. The multidrug transporter Pdr5 on the 25th anniversary of its discovery: An important model for the study of asymmetric ABC transporters (Review). Biochem J. 2015; 467: 353-363. Ref.: https://goo.gl/VzTCdc
- Egner R, Bauer BE, Kuchler K. The transmembrane domain 10 of the yeast Pdr5p ABC antifungal efflux pump determines both substrate specificity and inhibitor susceptibility. Mol Microbiol. 2000; 35: 1255-1263. Ref.: https://goo.gl/IKyhb4
- Li H, Chen Z, Zhang C, Gao Y, Zhang X, et al. Resistance reversal induced by a combination of fluconazole and tacrolimus (FK506) in Candida glabrata. J Med Microbiol. 2015; 64: 44-52. Ref.: https://goo.gl/sK7p7r
- Chang R, Hare A, Rich P. Treating cosmetically induced nail problems. Dermatol Ther. 2007; 20: 54-59. Ref.: https://goo.gl/ULCqQr
- Militello G. Contact and primary irritant dermatitis of the nail unit diagnosis and treatment. Dermatol Ther. 2007; 20: 47-53. Ref.: https://goo.gl/vwidDe
- Rieder EA, Tosti A. Cosmetically Induced Disorders of the Nail with Update on Contemporary Nail Manicures. J Clin Aesthet Dermatol. 2016; 9: 39-44. Ref.: https://goo.gl/pwVtda
- Chen AF, Chimento SM, Hu S, Sanchez M, Zaiac M, et al. Nail damage from gel polish manicure. J Cosmetic Derm. 2012; 11: 27-29. Ref.: https://goo.gl/Bhoqcp
- Jefferson J, Rich P. Update on nail cosmetics. Dermatol Ther. 2012; 25: 481-490. Ref.: https://goo.gl/YDzKYF