More Information
Submitted: August 03, 2024 | Approved: August 24, 2024 | Published: August 26, 2024
How to cite this article: Hillman AK, Ramis P, Nielsen P, Rohren EM. Establishment of a Best Practice Recommendation (BPR) for Abdominal Aortic Aneurysms in a Large Multi-State Radiology Practice: Adoption and Impact. J Clin Med Exp Images. 2024; 8(1): 007-012. Available from: https://dx.doi.org/10.29328/journal.jcmei.1001032.
DOI: 10.29328/journal.jcmei.1001032
Copyright License: © 2024 Hillman AK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Abdominal aortic aneurysms; Best practice recommendations; Quality Improvement
Establishment of a Best Practice Recommendation (BPR) for Abdominal Aortic Aneurysms in a Large Multi-State Radiology Practice: Adoption and Impact
Andrew K Hillman1, Phil Ramis2, Patrick Nielsen2 and Eric M Rohren1*
1Baylor College of Medicine, Texas, USA
2Radiology Partners Research Institute, California, USA
*Address for Correspondence: Eric M. Rohren, Baylor College of Medicine, Texas, USA, Email: [email protected]
Purpose of the study: To evaluate the performance of Best Practice Recommendation (BPR) compliance in reporting abdominal aortic aneurysm findings on imaging, comparing the results before and after its deployment.
Methods: Best Practice Recommendations for AAA were deployed in 2020 at a large radiology practice site. Reports between January 2018 through October 2022 were reviewed, representing studies read prior to and subsequent to the implementation of the reporting standards. Cases of abdominal aortic aneurysms ≥ 2.6 cm were counted by year. Adherence to the BPR for each year was calculated as [total number of confirmed cases of ≥ 2.6 cm AAAs with compliant reports] * 100 / [the total number of confirmed ≥ 2.6 cm AAAs]. A secondary analysis was performed to determine whether there was a statistically significant difference in the proportion of BPR-compliant reports for AAA cases before (from 2018 to 2019) and after (from 2020 to 2022) BPR deployment using a chi-square test.
Results: From January 2018 to December 2022, there were 8,693 reports referencing AAA. After excluding cases of suspected AAA (N = 2,131), confirmed AAAs with indeterminate sizes (N = 103), and confirmed AAAs with sizes < 2.6 cm (N = 85), the number of AAA cases ≥ 2.6 cm in size was 6,374. Concordance with the BPR standards for the remaining cases with sizes ≥ 2.6 cm were 1.6% and 4.1% in 2018 and 2019, respectively. Post-implementation of BPRs, there was a substantial improvement in guideline adherence to 32.1%, 84.3%, and 83.6% in 2020, 2021, and 2022, respectively.
In general, the proportion of BPR-compliant reports of AAA cases in the pre-deployment (3.6%) period statistically differs (p - value < 0.0001) from those in the post-deployment period (73.9%)
Conclusion: Adherence to reporting standards increased after the BPR deployment in 2020. The inclusion of management recommendations in the radiology report when AAA is identified is a simple and cost-effective way of improving outcomes for patients with AAAs through appropriate follow-up treatment.
An AAA is diagnosed when a permanent focal dilation is 50% greater than the relatively standard diameter of the adjacent healthy abdominal aorta [5]. Abdominal aortic aneurysms (AAA) are often found incidentally on imaging of asymptomatic patients for an unrelated condition [1]. Patients with larger AAAs (≥ 5.5 cm) are at a higher risk of rupture and mortality even with the emergency surgical intervention [2,3].
Some studies suggest that insufficient radiological surveillance of asymptomatic AAAs has been associated with an increased risk of AAA rupture and mortality [4]. In encountering abdominal aortic aneurysms in daily practice, radiologists can potentially improve outcomes through identification of the abnormality, and issue clear and concise recommendations for patient management. Radiology Partners utilizes a Best Practice Recommendations guideline for AAA to provide guidance to radiologists as they encounter incidental AAAs detected in imaging studies [4].
BPRs are quality improvement tools that, when properly implemented, help radiologists standardize recommendation reports in radiology practices, ensuring patients receive appropriate follow-up recommendations tailored to their specific conditions [4]. By doing so, BPRs prevent unnecessary and often costly follow-up procedures or imaging studies that are unlikely to benefit certain patients, preserving resources for those who genuinely need them [4]. This is especially important for conditions like AAA, where periodic follow-up procedures and monitoring of the patient’s AAA size over time may be required. It is critical that such guidelines be adhered to in order to appropriately guide patient management.
Purpose of study
To evaluate the performance of Best Practice Recommendation (BPR) compliance in reporting abdominal aortic aneurysms findings on imaging, comparing the results before and after its deployment.
Study Selection: Radiology reports for studies performed between 2018 and 2022 were obtained from the electronic medical records of a large radiology practice within Radiology Partners. Imaging modalities include ultrasound scan (US), computed tomography (CT) scan with and without contrast, magnetic resonance imaging (MRI) with and without contrast, and positron emission tomography (PET) with and without contrast. Natural language processing was utilized to identify reports referencing abdominal aortic aneurysms. Between January 2018 and December 2022, 8,693 AAA cases were identified. The study was determined to be exempt from review by the institutional review board at Baylor College of Medicine.
Deployment of BPR and determination of BPR adherence: For this study, the Clinical Value Team at RP introduced BPRs for reporting abdominal aortic aneurysms (AAAs) at this local practice on January 1, 2020. These BPRs were formulated by RP, based on the guidelines from the 2013 White Paper of the ACR Incidental Findings Committee II on Vascular Findings (Table 1) [5].
| Table 1. Radiology Partners BPR for AAA surveillance | |
| AAA sizes (cm) | Follow-up Recommendations1 |
| 2.6-2.9 | Every 5 years2 |
| 3.0-3.4 | Every 3 years |
| 3.5-3.9 | Every 2 years |
| 4.0-4.4 | Every 12 months, recommend vascular consultation |
| 4.5-5.4 | Every 6 months, recommend vascular consultation |
| ³5.5cm | Referral to a vascular specialist |
| *1AAA BPRs are based on ACR White paper: Journal of ACR 2013; 10(10): 789-794; 2For aortas of maximum diameter 2.6-2.9 cm meeting the criteria for AAA (³1.5 x proximal normal segment; no follow-up if <1.5 x proximal normal segment) | |
A Best Practice Recommendation (BPR) is a proprietary quality metric crafted by a group of radiologists and support staff after evaluating the latest white papers, patient outcomes, reimbursement criteria, and possible medicolegal concerns related to specific case types. Following multiple rounds of revisions and feedback, the BPR is finalized and made available for implementation at individual practices. Local practices that wish to adopt the BPR must undergo mandatory training.
Predictive text technology is integrated into the dictation software used for reporting imaging findings. When specific keywords are in the impression or findings sections of radiology reports, they are flagged by the predictive text tool, and the BPR guidelines automatically appear on the screen. This allows radiologists to determine whether a specific follow-up recommendation is necessary, based on the unique characteristics of the patient’s condition, such as the size of an AAA.
For qualifying cases, such as AAAs, radiologists’ reports are assessed using both natural language processing (NLP) tools and human reviewers, generating a monthly report on BPR adherence. These reports, which include detailed case-by-case insights, are shared with the radiologist and the local practice operations team to guide them for continuous improvement. The feedback provided helps radiologists understand why specific cases did or did not comply with BPR standards, enabling them to refine their follow-up recommendations in future reports to enhance adherence.
Inclusion or exclusion criterion: In this study, we first reviewed imaging reports and excluded those containing phrases like “suspected AAA” or “possible AAA.” Additionally, reports that did not specify the size of the AAA were excluded. Finally, studies with AAA findings of less than 2.6 cm were excluded, as they do not require follow-up according to BPR guidelines. Only AAAs measuring 2.6 cm or larger were included to focus on cases that actually require follow-up recommendations.
Statistical analysis
Radiology reports were reviewed to determine the size of the AAA detected, and whether the report and recommendations were compliant with the BPR or not compliant with the BPR.
Our primary analysis was to determine BPR adherence in each year, calculated as the [total number of confirmed cases of AAAs with sizes ≥ 2.6 cm that received BPR reports for AAA surveillance] * 100 / [total number of confirmed AAAs 2.6 cm in that same year].
A secondary analysis was performed to determine whether there was a statistically significant difference in the proportion of BPR-compliant reports for AAA cases before (from 2018 to 2019) and after (from 2020 to 2022) BPR deployment. We used a 2*2 contingency table to conduct a chi-square test for this analysis, with the a significance level set at 0.05 (Table 4). Our null hypothesis assumed no statistical difference in the proportions of BPR-compliant studies between the pre- and post-BPR deployment periods. The analysis was conducted using StataNow/BE version 18.5.
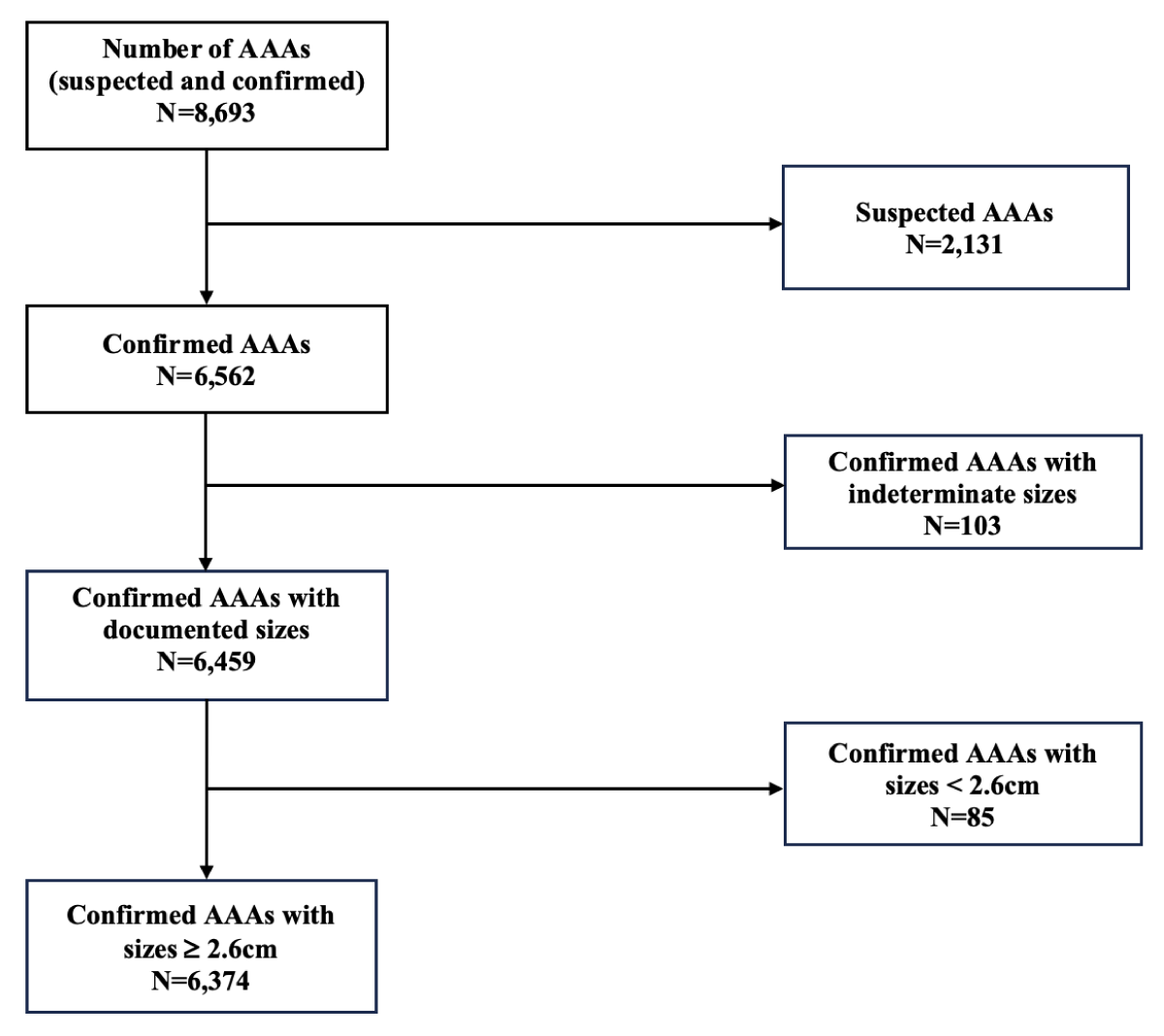
A total of 8,693 studies with AAA findings were retrieved from the practice under study. We excluded 2,131 studies labeled as “suspected AAA” or “possible AAA” and 103 studies where the AAA size was not mentioned in the report (Figure 1).
Figure 1: A flowchart showing AAAs cases from 2018 to 2022 that were excluded and included for BPR adherence evaluation.
Among the remaining cases of confirmed AAAs with documented sizes (N = 6,459), those with confirmed AAAs with sizes < 2.6 cm (N = 85) were excluded (Figure 1).
Out of the 6,374 confirmed AAA cases with sizes > 2.6 cm, 65.5% (4,173) had sizes between 2.6-3.9cm, 27.9% (1,777) had sizes between 4.0-5.4cm, and 6.7% (424) had sizes ≥ 5.5 cm.
Patients aged ≥ 75 contributed the most to AAA cases in all three AAA size categories; 48.5% (2,024) of patients with sizes 2.6 - 3.9 cm, 53.1% (944) of patients with sizes 4.0 - 5.4 cm, and 55.9% (237) of patients with sizes ≥ 5.5 cm. About 73.3% (4,674) of AAA cases with sizes ≥ 2.6 cm were reported in males.
About 61.6% (3,928) of the confirmed cases with sizes ≥ 2.6 cm had imaging studies done in the outpatient setting, 16.9% (1,080) in the in-patient setting, and 11.7% (748) were done in the emergency setting (Table 2). CT scan, with and without contrast, was the most used imaging modality (63.8%), followed by US scan (27.4%) (Table 2.). The least used imaging modalities were MRI scans (with and without contrast) (6.0%) and PET scans, with and without contrast (2.9%) (Table 2).
| Table 2. Different AAA size categories (with sizes ³ 2.6 cm) from 2018 to 2022 by age category, gender, place of imaging, imaging modality used, the type and location of AAA | ||||
| AAAs with sizes 2.6-3.9cm (N=4,173) |
AAAs with sizes 4.0-5.4cm (N=1,777) |
AAAs with sizes ³ 5.5cm (N=424) |
p-value | |
| Age Category, N (%) <45 45-54 55-64 65-74 ³ 75 |
12 (0.3) 48 (1.2) 444 (10.6) 1,645 (39.4) 2,024 (48.5) |
6 (0.3) 17 (1.0) 157 (8.8) 653 (36.8) 944 (53.1) |
3 (0.7) 5 (1.2) 49 (11.6) 130 (30.7) 237 (55.9) |
0.002 |
| Gender, N (%) Male Female unknown |
3,027 (72.5) 1,138 (27.3) 8 (0.2) |
1,329 (74.8) 447 (25.2) 1 (0.06) |
318 (75.0) 105 (24.8) 1 (0.2) |
0.258 |
| Place Of Imaging, N (%) Emergency Inpatient Outpatient unknown |
483 (11.6) 708 (17.0) 2,549 (61.1) 433 (10.4) |
215 (12.1) 295 (16.6) 1,119 (63.0) 148 (8.3) |
50 (11.8) 77 (18.2) 260 (61.3) 37 (8.7) |
0.290 |
| Imaging Modality*, N (%) US CT MRI PT |
1,232 (29.5) 2,571 (61.6) 261 (6.3) 109 (2.6) |
439 (24.7) 1,174 (66.1) 103 (5.8) 61 (3.4) |
74 (17.5) 323 (76.2) 15 (3.5) 12 (2.8) |
< .0001 |
| Type Of AAA, N (%) Fusiform Saccular Unreported |
887 (21.3) 136 (3.3) 3,150 (75.5) |
315 (17.7) 50 (2.8) 1,412 (79.5) |
82 (19.3) 6 (1.4) 336 (79.3) |
0.004 |
| Location Of AAA, N (%) Infrarenal Suprarenal Juxtarenal Pararenal Unreported |
1,875 (44.9) 22 (0.5) 5 (0.1) 5 (0.1) 2,266 (54.3) |
862 (48.5) 16 (0.9) 7 (0.4) 0 (0.0) 892 (50.2) |
219 (51.7) 7 (1.7) 6 (1.4) 3 (0.7) 189 (44.6) |
< .0001 |
| *US=Ultrasound scan; CT=Computed tomography scan with and without contrast; MRI=Magnetic resonance imaging with and without contrast; PT= Positron emission tomography. | ||||
1,284 cases were reported as fusiform-type AAAs (20.1%), and 192 cases were reported as saccular-type AAAs (3.0%) (Table 2). The remaining 4,898 (76.8%) cases did not report the type of AAA. 46.4% (2,956) of AAA cases were infrarenal, 0.7% (45) were suprarenal, 0.3% (18) were juxtarenal, and 0.1% (8) were pararenal. About 52.5% (3,347) of cases did not report the location of the AAA (Table 2).
BPR adherence by year
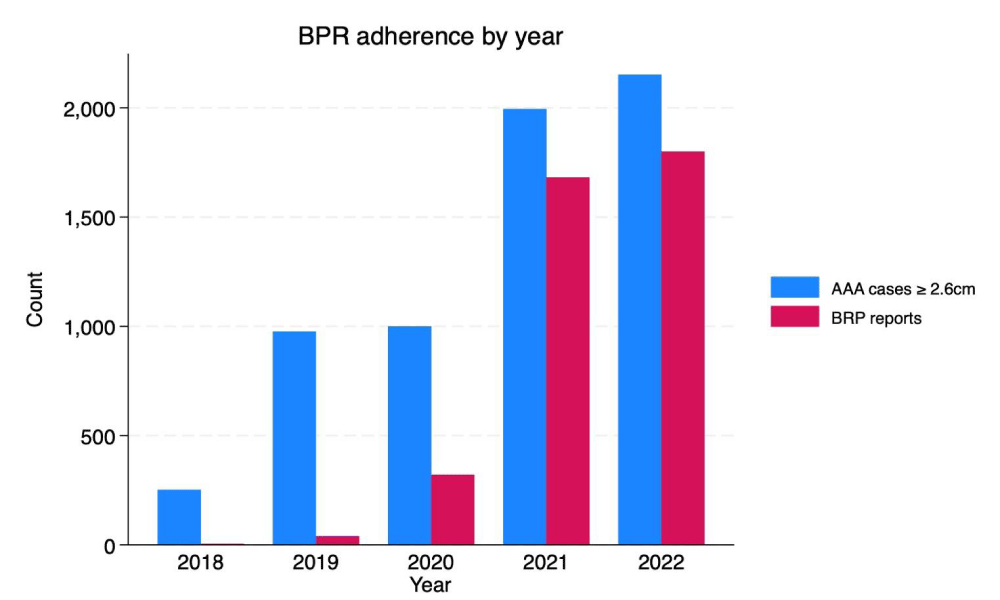
At the designated radiology practice, the confirmed cases of AAAs with sizes ≥ 2.6 cm were 252, 976, 1,000, 1,994, and 2,152 in 2018, 2019, 2020, 2021, and 2022, respectively (Table 3). AAA BPR adherence before deployment in January 2020 was 1.6% and 4.1% in 2018 and 2019, respectively. At the end of 2020, BPR adherence shot up to 32.1% (Table 3). BPR adherence improved to 84.3% in 2021 and 83.6% in 2022 (Table 3). A breakdown of the number of cases by AAA size category and the BPRs received each year are illustrated in Figure 2.
| Table 3. A breakdown of AAA cases by size category and BPR adherence from 2018 to 2022 | ||||||||||
| 2018 | 2019 | 2020 | 2021 | 2022 | ||||||
| Confirmed AAA size (cm) |
Number of Cases | BPR (%) | Number of Cases | BPR (%) | Number of Cases | BPR (%) | Number of Cases | BPR (%) | Number of Cases | BPR (%) |
| 2.6-3.9cm | 154 | 3 (2.0) | 569 | 24 (4.2) | 585 | 210 (35.9) | 1,409 | 1,247 (88.5) | 1,456 | 1,299 (89.2) |
| 4.0-5.4cm | 78 | 0 (0.0) | 321 | 4 (1.3) | 333 | 80 (24.0) | 483 | 375 (77.6) | 562 | 409 (72.8) |
| ³ 5.5cm | 20 | 1 (5.0) | 86 | 12 (14.0) | 82 | 31 (37.8) | 102 | 59 (57.8) | 134 | 92 (68.7) |
| Total | 252 | 4 (1.6) | 976 | 40 (4.1) | 1,000 | 321 (32.1) | 1,994 | 1,681 (84.3) | 2,152 | 1,800 (83.6) |
Figure 2: BPR adherence by year.
Pre- and post-BPR deployment period adherence
BPR compliance was 3.6% and 73.9% in the pre-and post-BPR deployment, respectively (Table 4). We reject the null hypothesis and conclude that the proportion of BPR-compliant reports of AAA cases in the pre-deployment period statistically differ significantly (p - value < 0.0001) from those in the post-deployment period (Table 4).
| Table 4: BPR adherence pre- and post-BPR deployment. | ||||
| BPR-compliant status of AAA cases | ||||
| Period | Non-BPR compliant | BPR compliant | Total | p - value |
| Pre-deployment (2018 to 2019) | 1184 (96.4) | 44 (3.6) | 1,228 | < 0.0001 |
| Pre-deployment (2020 to 2022) | 1344 (26.1) | 3802 (73.9) | 5,146 | |
| Total | 2, 528 (39.7) | 3,846 (60.3) | 6,374 | |
AAA develops when a permanent focal dilation is 50% greater than the relatively normal diameter of the adjacent healthy abdominal aorta [6]. About 90% of AAAs are fusiform and commonly located at the segment of the abdominal aorta below the origins of the renal arteries (infrarenal) [6]. Risk factors for the AAA include increasing age (with incidence peaking after age 60), male sex, race (more common in Caucasians, less common in Asians, African Americans, and Hispanics), hypertension, hypercholesterinemia, history of smoking, family history of AAA, atherosclerosis [6,1], and connective tissue diseases (Ehlers Danlos, Marfan syndrome). Our results show that AAA ≥ 2.6 cm tends to be more prevalent in males and patients aged ≥ 75 (Table 1).
It is common to find AAAs coexisting with other large vessel aneurysms, such as iliac artery aneurysms [1], and medium and small-sized vessel aneurysms, such as popliteal and intracranial cerebral aneurysms [7,8]. Most AAAs are usually asymptomatic and found incidentally on imaging during investigation for some other diseases [1]. The prevalence of all AAAs (including those with indeterminate sizes and those with size < 2.6 cm) from 2018 to 2020 in our study was about 0.56%, lower than the 4-8% prevalence reported in screening studies [6]. Since screening programs target high-risk individuals, the prevalence of the disease in the general population at presentation in the outpatient or inpatient setting is expected to be lower than the prevalence during screening.
AAAs tend to enlarge over time with an estimated growth rate of 0.2-0.3cm per year for 3-5cm AAAs and 0.3-0.5cm per year for >5cm AAAs [1]. The risk of AAA rupture increases with its increasing size [9]. Brown et al. found that the average risk of rupture in male and female patients with 5.0-5.5cm AAAs were 1.0% and 3.9% per year, respectively [2]. In male and female patients with AAA sizes ≥6.0, the average risk was 14.1% and 22.3% per year [2]. It is estimated that about 59-85% of patients with ruptured AAA die even before they are hospitalized or receive surgery [3]. Even with emergency AAA repair, the mortality risk post-surgery can be as high as 50%. In contrast, the 30-day mortality after an elective repair is about 3-5% [4]. In a study by Brox et al., emergency repair of a ruptured AAA was found to be more costly than elective repair in the United States and Canada [10]. In a different study in New Zealand, the average cost of an emergency repair of a ruptured AAA was $38,804, and $28,019 for the elective procedure [11]. Other prices, like laboratory investigations and blood products, were higher for those with emergency repairs than those receiving elective repairs [11]. This underscores the need for AAA screening and BPRs in radiologists’ reports for AAA surveillance.
The US Preventive Service Task Force recommends that men aged 65 to 75 with a smoking history do a 1-time screening for AAA with ultrasonography and selectively offer screening with ultrasonography in men in the same age group with no smoking history [12]. Also, to monitor AAA size growth, several bodies have released their version of recommendations based on the AAA size at the time of examination. The American College of Radiology (ACR), American College of Cardiologists, American Heart Associations, and Society of Vascular Surgery have released follow-up recommendations that ensure that AAAs are surveilled periodically and referred to vascular specialists when necessary. These follow-up recommendations differ slightly, such as how often the patient should be examined to assess new size. BPR implemented by the Clinical Value Team at RP follows recommendations based on the white paper of the ACR Incidental Findings Committee II on vascular findings [5].
The significance of the study was to assess the success or failure of the BPR program since its deployment by measuring BPR adherence. In this study, BPRs for AAA surveillance were deployed at a large radiological practice in January 2020. Before deployment, very few BPRs were included in radiologists’ reports (1.6% and 4.1% in 2018 and 2019, respectively). Post-deployment, the group saw substantial improvement in BPR-compliant reports for AAAs; 32.1% in 2020, 84.3% in 2021, and 83.6% in 2022. Despite this success, we would like to make a few recommendations to improve and standardize radiology reports for AAAs. In addition to BPRs for AAA surveillance and size reports, we recommend that radiologists clearly state the type and location of the aneurysm. The management of some AAAs depends on the type. The Society of Vascular Surgeon practice guidelines recommend that saccular AAAs be electively treated at a smaller diameter [13]. However, the exact minimum diameter to warrant elective repair has yet to be determined. Karthaus et al., in their study, recommended elective repair of saccular AAAs at smaller diameters (> 4.5 cm) than fusiform AAAs (> 5.5 cm) [14]. Our data shows that about 76.8% of AAA cases did not have information on the type of aneurysm, and about 52.5% did not have information on the location of the aneurysm. A complete report on a diagnosed AAA gives a clear picture of the pathology detected to the referring physician or vascular specialist and may influence management and treatment.
Study Limitation
Since this is a retrospective study, the data does not allow for patient-by-patient clinical follow-up to determine the impact of BPR inclusion in radiology reporting. Thus, patient outcomes, such as the risk of a future elective or emergency repair for those with large-sized AAAs, could not be determined. This research utilizes established guidelines for AAA management to infer clinical impact, but future studies would be needed to observe patient-specific outcomes.
Adherence to the Best Practices Recommendation (BPR) guidelines significantly improved after its deployment at the study practice, indicating the program’s success. Institution of a BPR program which includes specific guidelines for AAA description and management recommendations is a simple and cost-effective way of improving outcomes for patients with AAAs. By incorporating established information from the medical societies, the BPR effectively provides guidance to physicians whose patients have had AAA diagnosed on imaging. Further, a programmatic approach to BPR can establish standardization of reporting across a large radiology practice, elevating the level of care for all patients in a large network.
Data statement
The authors declare that they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis. The clinical data used to support the findings of this study are restricted by the Baylor College of Medicine Institutional Review Board in order to protect patient privacy. Data are available from Eric M. Rohren ([email protected]) for researchers who meet the criteria for access to confidential data.
Conflict of Interest: The authors declare that there are no conflicts of interest regarding the publication of this paper.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
- Shaw PM, Loree J, Gibbons RC. Abdominal Aortic Aneurysm. [Updated 2023 Mar 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470237/
- Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. Journal of Vascular Surgery. 2003 Feb;37(2):280-4. https://doi.org/10.1067/mva.2003.119
- D'Souza D, Weerakkody Y, Alhusseiny K. Abdominal aortic aneurysm [internet]. Radiopaedia.org. [cited 2023 March 28]. Available from: https://radiopaedia.org/articles/abdominal-aortic-aneurysm?lang=us
- Ahmed S, Mitsky J, Rawal U, Sheth S, Bronner J. Asymptomatic Abdominal Aortic Aneurysm: Standardizing Reporting Recommendations at a Large Multistate Radiology Practice. Journal of the American College of Radiology. 2021 Sep;18(9):1317-23. https://doi.org/10.1016/j.jacr.2021.04.009
- Khosa F, Krinsky G, Macari M, Yucel EK, Berland LL. Managing Incidental Findings on Abdominal and Pelvic CT and MRI, Part 2: White Paper of the ACR Incidental Findings Committee II on Vascular Findings. Journal of the American College of Radiology. 2013 Oct;10(10):789-94. https://doi.org/10.1016/j.jacr.2013.05.021
- Chung J. Epidemiology, risk factors, pathogenesis, and natural history of abdominal aortic aneurysm [internet]. UptoDate.com. [cited 2023 March 28]. Available from: https://www.uptodate.com/contents/epidemiology-risk-factors-pathogenesis-and-natural-history-of-abdominal-aortic-aneurysm
- Dent TL. Multiple Arteriosclerotic Arterial Aneurysms. Archives of Surgery, 1972 Aug 1;105(2):338 https://doi.org/10.1001/archsurg.1972.04180080184031
- Rouchaud A, Brandt M, Rydberg A, Kadirvel R, Flemming K, Kallmes D, Brinjikji W. Prevalence of Intracranial Aneurysms in Patients with Aortic Aneurysms. AJNR American Journal of Neuroradiology. 2016 Sep;37(9):1664-8. https://doi.org/10.3174/ajnr.a4827
- Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: A comprehensive review. Experimental & Clinical Cardiology, 2011;16(1):11-5. https://pubmed.ncbi.nlm.nih.gov/21523201/
- Brox AC, Filion KB, Zhang X, Pilote L, Obrand D, Haider S, Azoulay A, Eisenberg MJ. In-Hospital Cost of Abdominal Aortic Aneurysm Repair in Canada and the United States. Archives of Internal Medicine, 2003 Nov 10;163(20):2500. https://doi.org/10.1001/archinte.163.20.2500
- Peek KN, Khashram M, Wells JE, Roake JA. The costs of elective and emergency abdominal aortic aneurysm repair: a comparative single centre study. New Zealand Medical Journal, 2016 Apr 22;129(1433):51-61. https://pubmed.ncbi.nlm.nih.gov/27349161/
- Abdominal aortic aneurysm: Screening. (2019, December 10). Uspreventiveservicestaskforce.org; US Preventive Services Taskforce. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/abdominal-aortic-aneurysm-screening
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery. 2018 Jan;67(1):2-77.e2. https://doi.org/10.1016/j.jvs.2017.10.044
- Karthaus EG, Tong TML, Vahl A, Hamming JF. Saccular Abdominal Aortic Aneurysms. Annals of Surgery. 2019 Nov;270(5):852-8. https://doi.org/10.1097/sla.0000000000003529