Abstract
Case Report
Tumours of the Uterine Corpus: A Histopathological and Prognostic Evaluation Preliminary of 429 Patients
Jorge F Cameselle-Teijeiro*, Javier Valdés-Pons, Lucía Cameselle-Cortizo, Isaura Fernández-Pérez, MaríaJosé Lamas-González, Sabela Iglesias-Faustino, Elena Figueiredo Alonso, María-Emilia Cortizo-Torres, María-Concepción Agras-Suárez, Araceli Iglesias-Salgado, Marta Salgado-Costas, Susana Friande-Pereira and Fernando C Schmitt
Published: 30 January, 2017 | Volume 1 - Issue 1 | Pages: 011-019
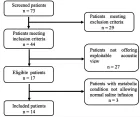
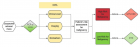
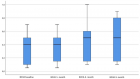
A histopathological review preliminary of 429 patients diagnosed with tumours of the uterine corpus (TUC) cancer between 1984- 2010 in the Vigo University Hospital Complex (Spain) were evaluated prospectively for over 5 years. Of these 403 (93.9%) were epithelial tumours: 355 (82.7%) were adenocarcinomas of the endometrioid type, 5 (1.1%) mucinous adenocarcinoma, 10 (2.3%) serous adenocarcinoma, 17 (3.9%) clear cell carcinomas, 11 (2.5%) mixed adenocarcinoma, 4 (0.9%) undifferentiated carcinomas and 1 (0.2%) squamous cell carcinomas. A total 20 (4, 6%) were mesenchymal tumours: 4 (0.9%) endometrial stromal sarcoma, 7 (1.6%) Leiomyosarcoma, 9 (2%) Mixed endometrial stromal and smooth muscle tumour. A total 1 (0.2%) were mixed epithelial and mesenchymal tumours: (0.2%) Adenosarcoma 1. And 5 (1.1%) were Metastases from extragenital primary tumour (3 carcinomas of the breast, 1 stomach and 1 colon). The mean age at diagnosis from total series were 65, 4 years (range 28-101 years). Age was clearly related to histologic type: Endometrial stromal sarcoma 46.0 years, Leiomyosarcomas 57.1 years, Adenocarcinomas of the endometrioid type 65.4 years, Clear cell carcinomas 70.1 years and mixed endometrial stromal and smooth muscle tumours 71.2 years. Five-year disease-free survival rates for the entire group were: Endometrial stromal sarcoma 50%, Leiomyosarcomas 28.6%, Adenocarcinomas of the endometrioid type 83.7%, Clear cell carcinomas 64.7% and mixed endometrial stromal and smooth muscle tumours 44.4%. The 5-year disease-free survival rates of patients with Adenocarcinomas of the endometrioid type tumors were 91.4% for grade 1 tumors, 77.5% for grade 2, and 72.7% for grade 3.
In conclusion, we describe 5-year histological and disease-free survival data from a series of 429 patients with TUC, observing similar percentages to those described in the medical literature. The only difference we find with other published series is a slightly lower percentage of serous carcinomas (ESC) that the Western countries but similar to the 3% of all ESC in Japan. Our investigation is focus at the moment on construct genealogical trees for the possible identification of hereditary syndromes and to carry out germline mutation analysis.
Read Full Article HTML DOI: 10.29328/journal.jcmei.1001004 Cite this Article Read Full Article PDF
Keywords:
Tumours of the uterine corpus; Endometrial cancer; Uterine serous carcinoma; Epidemiology
References
- Kurman RJ, Carcangiu ML, Herrington CS and Young RH: WHO classification of tumours of female reproductive organs. WHO Classification of tumours. 6: (4TH). (Lyon). IARC Press. 122-167. 2014.
- Rose PG. Endometrial Carcinoma. N Engl J Med 1996; 335: 640-649. Ref.: https://goo.gl/fIWSnz
- Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008; 198: 1-6. Ref.: https://goo.gl/VBuv4W
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983; 15:10-17. Ref.: https://goo.gl/2Jsq5S
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. The Lancet 2014; 7: 268-278. Ref.: https://goo.gl/L7tEr6
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. The Lancet. 2016; 387: 1094-1108. Ref.: https://goo.gl/B4M4qx
- Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009; 16: 8-13. Ref.: https://goo.gl/NLz8Lh
- Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, et al. Molecular pathology of endometrial carcinoma: Practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol. 2009; 62: 777-785. Ref.: https://goo.gl/gxXjZy
- Clarke BA, Gilks CB. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol 2010; 63: 410-415. Ref.: https://goo.gl/bW3JHk
- Talhouk A, McAlpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016; 3: 14. Ref.: https://goo.gl/5E7ysZ
- Abeler VM, Kjørstad KE, Berle E. Carcinoma of the endometrium in Norway: a histopathological and prognostic survey of a total population. Int J Gynecol Cancer. 1992; 2: 9-22. Ref.: https://goo.gl/Y5xguK
- Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control. 2009; 16: 46-52. Ref.: https://goo.gl/0ZVd1Y
- Cirisano FD, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, et al. Epidemiologic and Surgicopathologic Findings of Papillary Serous and Clear Cell Endometrial Cancers When Compared to Endometrioid Carcinoma. Gynecologic Oncology. 1999; 74: 385-394. Ref.: https://goo.gl/b7Bwuc
- Lobo FD, Thomas E. Type II endometrial cancers: A case series. J Midlife Health. 2016; 7: 69-72. Ref.: https://goo.gl/nNy3pY
- Black C, Feng A, Bittinger S, Quinn M, Neesham D, et al. Uterine Papillary Serous Carcinoma: A Single-Institution Review of 62 Cases. International Journal of Gynecological Cancer. 2016; 26: 133-140. Ref.: https://goo.gl/N67WyA
- Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 2006; 94: 642-646. Ref.: https://goo.gl/hWmQNS
- Annual report ofendometrial cancer, 2008. Acta Obstet Gynaecol Jpn. 2010; 62: 853-876.
- Hoang LN, McConechy MK, Kobel M, Han G, Rouzbahman M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. 2013; 37: 1421-1432. Ref.: https://goo.gl/T3fx7t
- Lax SF, Kurman RJ. A dualistic model for endometrial carcinogenesis based on immunohistochemical and molecular genetic analyses. Verh Dtsch Ges Pathol. 1997; 81: 228-232. Ref.: https://goo.gl/wwuzjc
- McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012; 228: 20-30. Ref.: https://goo.gl/7Hw7Hl
- Alvarez T, Miller E, Duska L, Oliva E. Molecular profile of grade 3 endometrioid endometrial carcinoma: is it a type I or type II endometrial carcinoma? Am J Surg Pathol. 2012; 36: 753-761. Ref.: https://goo.gl/ShXOXw
- Coenegrachts L, Garcia-Dios DA, Depreeuw J, Santacana M, Gatius S, et al. Mutation profile and clinical outcome of mixed endometrioid-serous endometrial carcinomas are different from that of pure endometrioid or serous carcinomas. Virchows Archiv. 2015; 466: 415-422. Ref.: https://goo.gl/RkRpLF
- Alkushi A, Kobel M, Kalloger SE, Gilks CB. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol. 2010; 29: 343-350. Ref.: https://goo.gl/4FLcP1
- Santacana M, Maiques O, Valls J, Gatius S, Abo AI, et al. A 9-protein biomarker molecular signature for predicting histologic type in endometrial carcinoma by immunohistochemistry. Hum Pathol. 2014; 45: 2394-2403. Ref.: https://goo.gl/ZC2fPF
- Kumar MB, Hart WR. Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer 1982; 50: 2163-2169. Ref.: https://goo.gl/soUMd6
- Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Broaddus R. Hereditary cancer panel testing in an unselected endometrial carcinoma cohort. Gynecologic Oncology 2016; 141:10-11. Ref.: https://goo.gl/QIZTXC
- Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh NU, et al. Broaddus R. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Modern Pathology 2016; 29: 1381-1389. Ref.: https://goo.gl/Uxjj44
- Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, et al. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol 2010; 116: 516-521. Ref.: https://goo.gl/mhtJai
- Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 2006; 66: 7810-7817. Ref.: https://goo.gl/U0NrcH
- Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 2007; 25: 5158-5164. Ref.: https://goo.gl/pec1c3
- Ketabi Z, Mosgaard BJ, Gerdes AM, Ladelund S, Bernstein IT. Awareness of endometrial cancer risk and compliance with screening in hereditary nonpolyposis colorectal cancer. Obstet Gynecol 2012; 120: 1005-1012. Ref.: https://goo.gl/TETYXw
- Gruber SB, Thompson WD. A population-based study of endometrial cancer and familial risk in younger women. Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev 1996; 5: 411-417. Ref.: https://goo.gl/x18XFJ
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003; 348: 919-932. Ref.: https://goo.gl/Ek9tjL
- Barrow E, Hill J, Evans DG. Cancer risk in Lynch Syndrome. Fam Cancer 2013; 12: 229-240. Ref.: https://goo.gl/1YWbL3
- Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 2006; 66: 7810-7817. Ref.: https://goo.gl/xIAAKx
- Hampel H, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Comment on: Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res 2007; 67: 9603. Ref.: https://goo.gl/tJtwum
- Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, et al. Implementation of Tumor Testing for Lynch Syndrome in Endometrial Cancers at a Large Academic Medical Center. Gynecol Oncol 2013; 130: 121-126. Ref.: https://goo.gl/lXddqC
- Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, et al. Prevalence of Lynch Syndrome among Patients with Newly Diagnosed Endometrial Cancers. PLoS One. 2013; 8: e79737. Ref.: https://goo.gl/q2vFt6
Figures:

Figure 1

Figure 2

Figure 3
Similar Articles
-
Tumours of the Uterine Corpus: A Histopathological and Prognostic Evaluation Preliminary of 429 PatientsJorge F Cameselle-Teijeiro*,Javier Valdés-Pons,Lucía Cameselle-Cortizo,Isaura Fernández-Pérez,MaríaJosé Lamas-González,Sabela Iglesias-Faustino,Elena Figueiredo Alonso,María-Emilia Cortizo-Torres,María-Concepción Agras-Suárez,Araceli Iglesias-Salgado,Marta Salgado-Costas,Susana Friande-Pereira,Fernando C Schmitt. Tumours of the Uterine Corpus: A Histopathological and Prognostic Evaluation Preliminary of 429 Patients . . 2017 doi: 10.29328/journal.jcmei.1001004; 1: 011-019
Recently Viewed
-
Deep Learning-Powered Genetic Insights for Elite Swimming Performance: Integrating DNA Markers, Physiological Biometrics and Performance AnalyticsRahul Kathuria,Reeta Devi,Asadi Srinivasulu*. Deep Learning-Powered Genetic Insights for Elite Swimming Performance: Integrating DNA Markers, Physiological Biometrics and Performance Analytics. Int J Bone Marrow Res. 2025: doi: 10.29328/journal.ijbmr.1001020; 8: 006-015
-
Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2)Denise HM Bodden*,Yvonne Stikkelbroek,Daan Creemers,Sanne PA Rasing,Elien De Caluwe,Caroline Braet. Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2). Insights Depress Anxiety. 2025: doi: 10.29328/journal.ida.1001047; 9: 028-039
-
Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of DurabilityAyoub Mamad*,Mohammed Amine Bibat,Mohammed Amine Elafari,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of Durability. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001040; 10: 006-009
-
Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case ReportAyoub Mamad*,Mohammed Amine Elafari,Mohammed Amine Bibat,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case Report. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001039; 10: 003-005
-
Could metabolic risk factors contribute to the development of cervical cancer?Maydelín Frontela-Noda*,Eduardo Cabrera-Rode,Maite Hernández-Menéndez,Raquel Duran-Bornot. Could metabolic risk factors contribute to the development of cervical cancer?. Ann Clin Endocrinol Metabol. 2019: doi: 10.29328/journal.acem.1001011; 3: 001-006
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."